
By Bob Goemans

Hopefully you have read Part I in last months FAMA as it would be very helpful to understand what you're about to read here. Without its interpretations of various words, some of what is here may be misunderstood.
Over the past few years the thought has been that the difference between a plenum system and a Deep Sandbed (DSB) was that the plenum method functions via diffusion, and the DSB functions via bioturbation. This is false or perhaps just misplaced thinking. Both systems have diffusion and bioturbation. However, it's the degree of each that helps set them apart, along with some quite remarkable differences as noted below. Therefore each varies as to its biophysical rules and those in turn mostly depend upon the physical size of its substrate grains, its depth and whether or not the bed rests directly on the aquarium bottom or an elevated platform. A key word here would be 'Permeability.'
It could be said that no matter what the type or size sand grains or their depth, the microbial population will be in equilibrium with its supply of foodstuffs. But that is somewhat misleading because in the closed system it's the overall efficiency of the bed in relation to the systems incoming foodstuffs that really counts. Nitrogen pathways react differently to high or low levels of nitrate in the bulk water. If the microbial mediators are in equilibrium, they will respond nicely to either of those conditions. Yet, excesses occur for a reason! There may be a lack of balance or useful microbial mediators. Short-circuiting of the balancing act, e.g., excess oxygen inhibiting denitrification or the inverse relationship between that of oxygen concentration in the overlying water and the overall rate of denitrification when the water is rich in nitrates is another possibility.
Of course the interplay of infauna (bioturbation) can change the overall effectiveness of the bed in relation to bulk water nutrient excesses by creating additional anoxic areas. Yet, the predictability of infauna verses that of microbial processes does remain questionable. How all of this applies to the plenum method or DSB would depend upon the volume and composition of incoming nutrients and the health, condition, and type of bed. Regardless, for the reasons explained in Part I and below, a bed having more anoxic than anaerobic volume area, where a greater volume of more efficient microbes exist, (as explained below) would be able to respond very rapidly to an excessive bulk water nutrient flux. In addition, their existence and capability does not encompass guesswork that would accompany that of infauna mix, size, type, and distribution.
The Jaubert plenum method does have a remarkable difference from that of a DSB and that's the value of the electrical charge that accompanies matter in the depth of its bed. This charge is measured in millivolts (mV). Even though the mechanisms and pathways associated with sandbeds are quite involved, it could generally be said that sandbeds of any type are basically a chemical sink where the diffusion of nutrients through them are influenced by electrical charge. And that positive charges are attracted to negative charges. Accordingly, the water's surface and the air above are a negative mV. In the bulk water of the aquarium there is many charged molecules. Much of it is a positive mV. So is most of the living biomass, e.g., corals and fishes. Substrate surfaces are largely a negative mV. The sandbed itself is negative with increasing magnitude with depth. The deeper the sand, the more negative it becomes and the more positive charged nutrients are naturally attracted to lower depths. In the DSB, unless there is sufficient and effective bioturbation there could easily be accumulation of these nutrients as the most negative charge is the sand at the aquarium's bottom.
However, the plenum's sandbed differs radically from the DSB in this respect as the 'plenum space' beneath its bed actually retains a small amount of oxygen. This was actually tested by Sam Gamble and shown to usually stay slightly above .5 mg/l. The ability to retain some oxygen appears to keep the majority of the bed above in what we have defined in Part I as an 'anoxic' condition. And because of this minute oxygen content it is reasonable to assume the contents of the 'space' may have a less negative charge than the sand above and use its oxygen for the purpose of electron transfer. Therefore, yet to be oxidized compounds in the plenum space present a favorable condition for the microbes living on the sand particles above. In our opinion, this can be considered a natural supply and demand process that retrieves constituents from the plenum reservoir. How much accumulates there seems to depend upon system bioload and we known of no leaching of these excesses back into the bulk water unless there are major disturbances to the bed.
Another interesting finding from our research and which fits nicely with the ability of a plenum system to maintain more anoxic than anaerobic bed area is there are two different denitrification processes each having its own class of anaerobic bacteria. Assimilatory denitrification occurs in anaerobic areas (as defined in last month's article) and is where nitrate is reduced 'only' to that of ammonium, no further. It's accomplished by obligate anaerobic heterotrophs, and in fact they die if exposed to oxygen. Dissimilatory denitrification occurs in zones having a small amount of oxygen, again defined in last month's article as the anoxic zone. It's accomplished by facultative anaerobic heterotrophs and fully oxidizes nitrate back to nitrogen gas. One of the nice things about them is they are capable of living in areas containing little or no dissolved oxygen. They are enormously more efficient than the microbes living in the anaerobic zones!
In fact, when an energy source like glucose is arbitrarily added, they have an adenosine triphosphate (ATP) yield of approximately 34 times that of obligate anaerobic heterotrophs. The difference in energy unit yield of ATP corresponds to how much faster and more efficiently nutrients are reduced to energy. What's of great value here is if the majority of the sandbed can be kept in an anoxic state excessive bulk water nitrate levels can potentially be controlled because microbes actively strive to stay in equilibrium with the available food supply. If the majority of the bed is anaerobic, ammonium is the denitrification end product, not nitrogen gas. Therefore, another nitrogen compound of more significance is resulting! In the DSB where intervention of infauna exist, oxygen levels no doubt vary. But one has to wonder how well those anoxic areas are dispersed throughout the bed as actual measurements are sparse and the existences of accurate models do not exist.
In the wild, the anoxic layer is often quite shallow, possibly a layer only a few millimeters deep. Tidal movements help keep its water free of nutrient excesses. If we were to take a cross-section of that benthic sediment anoxic zone and count its number of microbes, one would say there's a huge number of microbes per unit of size. That could be considered a huge efficiency factor. But closed systems differ and nutrient excesses are often an on-going situation without benefit of tidal cleansing. One can not say with any assurances that one bed method, i.e., DSB or Jaubert plenum will outperform the other because of many variables missed by oversight and design. Yet, it would be fair to say from case histories that when a plenum space is created, the depth of its anoxic zone is extended and assured. The potential is now greater for increasing in astronomical terms the number of facultative anaerobic heterotrophs. And, when increased microbial efficiency 'and' some bioturbation are combined, it's an environment that appears to have extraordinary benefits, and very possibly fewer problematic variables.
It has been said it's impossible to have oxygen in the plenum (space) under a four inch bed of sand with grains in the range of 1 - 4 mm unless its brought there by the tunneling activity of infauna. Nonetheless, actual tests to plenum areas with only microbial colonies in its sandbed have confirmed its existence. Yet, in all honesty, how it gets there is still somewhat a mystery. There are some very real possibilities as noted by Dr. Craig Jones, a biochemist consultant for many large companies and various governmental entities.
We think number one is probably the main source of oxygen in the plenum, however, three is another contributor, with number two a possible contributor. If there's a point to be made here, its that oxygen 'is' present in the plenum and it's clearly not there only because of infauna.
Another area of interest between that of course sand or mud-like deep beds come from that of 'carbon' availability. Autotrophs, such as cyanobacteria, are those bacteria that utilize light and carbon dioxide to carry out their biological processes and can quickly use an abundance of inorganic carbon. Heterotrophs are mostly responsible for breaking down organic matter and thrive in areas where diffusion abounds and where organic carbon is well cycled. It is also a fact that mediating biochemical transformations (protein/enzymes) and genetic controls (DNA/RNA) show a common reliance on specific ratios of carbon (DOC), nitrogen (DON), and phosphorous (DOP). It could then be said organic carbon is a key player in how well inorganic nutrients, e.g., nitrogen and phosphorous are utilized. And, there appears to be a specific ratio needed, which is thought to be approximately thirty-six parts carbon, six parts nitrogen, and one part phosphorous, sometimes referred to as the Redfield Ratio.
Recent evidence suggests that when heterotrophic bacteria are limited by both organic carbon and mineral nutrients, they have a negative impact on their trophic neighbors in the microbial food web. In other words, if they suffer, it appears to negatively affect neighboring processes. In such situations, infauna may become a governing force. Again, whether that's a positive or negative depends upon their mix, type, quantity and distribution. Nevertheless, nitrogen is generally the primary limiting nutrient in marine systems because it controls the rate of primary production. If the system is supplied with high levels of 'nitrogen,' algal blooms generally occur.
Whether organic carbon is cycled or stored appears to be a matter that relates to how the sediment supplies heterotrophs and autotrophs their essential foodstuffs. In fact, it has been shown that when only an organic carbon source is added; autotrophs are out competed by heterotrophs for inorganic nutrients, demonstrating a need for the corresponding nitrogen. If inorganic nutrients are only added, autotrophs increase, such as cyanobacteria. Therefore, the ratios between carbon and nitrogen and that of phosphorous are very important factors when facilitating population densities of either bacterium. One thing for sure, shallow course-grained sandbeds along with where diffusion is the key player, are very efficient at cycling organic carbon so as to balance the ratio of available constituents.
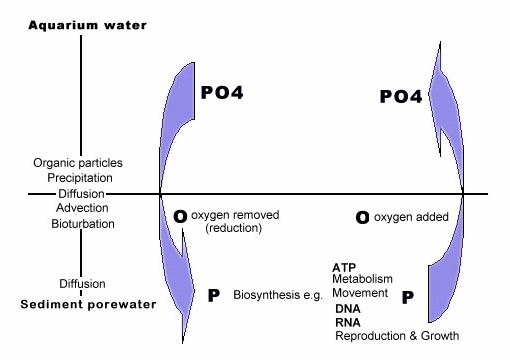
One last subject that is always on aquarists' minds is that of phosphate. Actually, most phosphate in our aquariums is due to the food fed and the quality of tap water used for evaporation makeup or water changes. However, its been said anaerobic areas, where obligate anaerobic heterotrophs reside, accumulate phosphate. As a matter of fact, the anaerobic area with its lower pH and redox is an efficient user of the oxygen electrons tied to the phosphorous element; therefore phosphate is quickly reduced to other phosphorus molecules and ions. That's a plus for the DSB as the majority of its substrate is usually in an anaerobic state.
It could then be said phosphate accumulates anywhere where its not attacked for its oxygen suggesting that in more aerobic and anoxic bed areas there would be greater accumulation since oxygen is readily available. However, that's also not accurate! In those areas it's mostly bound to calcium and manganese where it's quite stable because it is very easy to maintain its 'charge' balance. Therefore phosphate is usually not available for uptake in substrates unless associated with reducing conditions.
On the other hand, where there is infauna, whether that is in a DSB or plenum bed, they depend on getting oxygen to live and have to link with the substrate surface, whereas microbes do not. The tunneling processes (irrigation) of infauna can bring phosphate to the bed surface for other forms of bio-circulation. Yet, even though feasible it would depend upon the size and type of infauna, e.g., large worms. Nevertheless, when it does occur its in the form of orthophosphate, something not registered on aquarium phosphate test kits and could help cause phytoplankton and algae blooms. Keep in mind infauna also ingest sources of phosphate and produce phosphate-laden wastes, however, they should be considered more movers of the compound than users. (See Sketch Two)
Hopefully, we attained our goal, which was to get everyone on a level playing field and get them 'first' thinking more about the aquariums foundation - its microbial and infauna processes!
Let us leave you with an interesting statement, and then a quote recently sent to us from Dr. Jean Jaubert:
"As in any environment, oxygen is not only responsible for the direct mineralization of organic matter, but also for the reoxidation of the reduced electron acceptors from anaerobic respiration processes. Therefore, the relative position of the oxic-anoxic interface causes a layering of microbial processes which directly or indirectly depend on oxygen, and the basic principle controlling the flow of electrons from organic matter to oxygen is molecular diffusion! (Brune A., Frenzel P., & Cypionka H. 2000)"
"I believe that a nearly complete recycling can be achieved in a reef tank equipped with a plenum. A coral community has been thriving for 5 years without any change of water in the tank I have been keeping for 17 years at the University of Nice. The tank had only 3 inputs: light, food and freshwater (compensation for evaporation). (Jaubert, 2002, per.com.)"
Whatever is learned from these articles, the fact remains that grain size and depth play a major role in the class of bacteria that inhabit the bio-geochemical pathways of our sandbeds. And that bacteria are more predictable in our humble opinion than infauna. Nevertheless, when the right percentages of each are present, the substrate world has a very positive effect on the bulk water world! Whatever method is chosen, depends on how you see the value of the facts presented above.
References
Brune A., Frenzel P., & Cypionka H. 2000. Life in the oxic-anoxic interface: microbial activities and adaptations. FEMS Microbiology Reviews.
Craig, J.R., D.J. Vaughan, and B.J. Skinner. 1988. Resources of the Earth. Prentice-Hall: Englewood Cliffs, NJ.
Gamble, S. & Goemans, B. 2001. The New Wave, Aquarium Husbandry, A More Natural Approach. www.keysmariculture.com
Holtan, H., L. Kamp-Nielson, and A. O. Stuanes. 1988. Phosphorus in sediment, water, and soil: an overview. Hydrobiologia 170:19-34.
Jacquet S., Havskum H., & Thingstad T.F. 2002. Effects of Inorganic and Organic Nutrient Addition on a Coastal Microbial Community. Marine Ecology Progress Series, Vol. 228, 3-14.
Jaubert J., 1989. An integrated nitrifying-denitrifying biological system capable of purifying seawater in a closed circuit aquarium. Bull. Inst. Océanogr. Monaco. 5: 101-106.
Jaubert J., Marchioretti M., Priouzeau F., 1995. Carbon and calcium budgets in a semi-closed coral mesocosm. In: Proceedings of the 7th International Coral Reef Symposium, 289-293 (Boston, USA: April 1993).
Jaworski, N. A., O.Villa, Jr. 1981. A Suggested Approach for Developing Estuarine Water Quality Criteria for Management of Eutrophication. p. 499 in Estuaries and Nutrients, Nielsen, B.J., and L.E. Cronin (eds.) 1981. Humana Press: Clifton, NJ.
NOAA/EPA. National Oceanic and Atmospheric Administration and Environmental Protection Agency. 1988. Strategic Assessment of Near Coastal Waters, Chapter 3, Susceptibility and Concentration Status of Northeast Estuaries to Nutrient Discharges. NOA A: Washington, D.C.
Paerl, H.W., M.L. Fogel, P.W. Bates. 1993. Atmospheric Nitrogen Deposition in Coastal Waters: Implications for Marine Primary Production and C Flux. Trends in Microbial Ecology. P 459-464.
Rysgaard, S., Risgaard-Pertersen, N.,Sloth. N.P., Jensen,, K., and Nielsen,L.P.(1994) Oxygen regulation of nitrification and denitrification in sediments. Limnol. Oceanogr. 39, 1643-1652.
Smith, R.L. 1990. Ecology and Field Biology. 4th ed. Harper Collins Publishers, NY.
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/group5.html The Chemistry of Nitrogen and Phosphorous
http://www.bact.wisc.edu/microtextbook/Metabolism/OtherAssim.html Sulfur and Phosphate,©2000 Timothy Paustian, University of Wisconsin-Madison